Description
The ReStore Exo-Suit was developed in collaboration with the Wyss Institute at Harvard that was first announced more than three years ago: (Wyss Institute and ReWalk Robotics Form Collaboration, May 2016) This combined effort pairs the technological know-how of the Wyss Institute in the realm of soft exoskeletons (exosuits) and ReWalk’s depth of knowledge when it comes to clinician involvement.
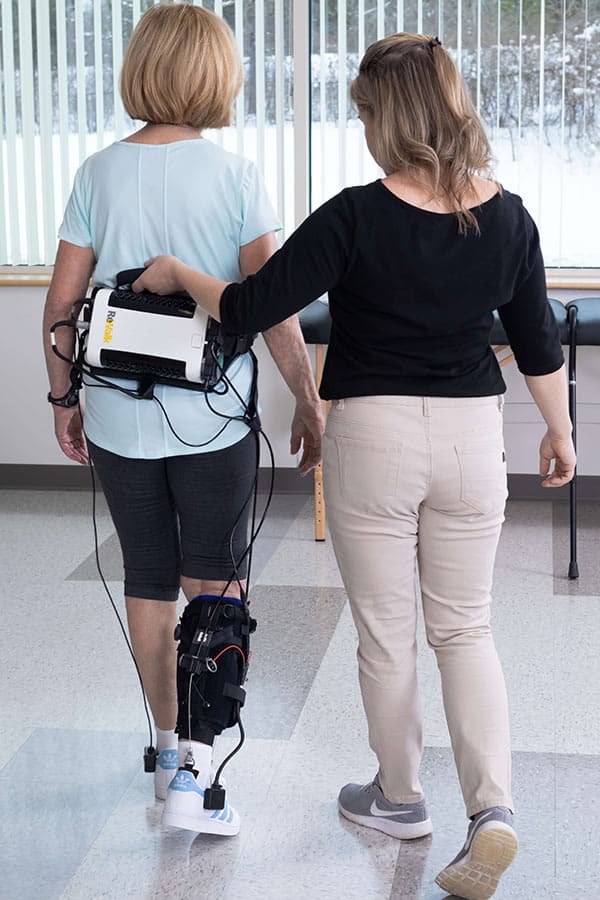
The ReStore exosuit aims to bring post-stroke gait training with a solution that is lighter and more nimble than a rigid lower body-powered exoskeleton. The exosuit is a data-driven, powered, gait rehabilitation device designed to facilitate functional gait training. It is also envisioned to have a significantly lower cost than a rigid frame gait assist exoskeleton.
The ReStore Exo-Suit becomes the first soft powered exoskeleton to be cleared by the FDA, and it also has an CE mark for distribution in Europe as well. The FDA approval is based on a multi-center clinical study, with the data generated used for the successful 510(k) submission.
Key attributes:
- Battery-powered ankle exosuit
- plantarflexion and dorsiflexion assistance
- adaptive control to synchronize with user’s gait
- small device footprint makes it compatible with additional support aids
Videos:
ReStore Exo-Suit for Stroke Rehabilitation-3 Modes of Function
Restore Exosuit From ReWalk Robotics | Rehabilitation Technology
Lifeward, formerly ReWalk Robotics (and Argo Medical Technologies before), Offices in Massachusetts, USA; Berlin, Germany; Yokneam Ilit, Israel, website
Exoskeleton Report does not endorse one exoskeleton product over another. The exoskeleton catalog is purely for educational purposes. The catalog is meant to provide an easily accessible birds-eye view of the exoskeleton industry, and a quick method to sort exoskeletons by type and purpose. All prices are approximate and are meant to provide a general sense of the cost of the devices.










Reviews
There are no reviews yet.