Description
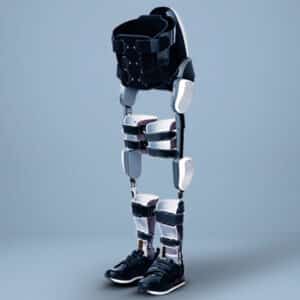
The EksoNR is a physical and neurorehabilitation tool intended for use in a clinical setting. Ideally, the training and practice with this powered exoskeleton will allow the users to “walk out of the device and back into their communities” faster.
The EksoNR retains all of the advances from the previous model and adds some new features as well. Ekso Bionics is continuously improving on its largest device and it now features:
- Data capture with session-specific metrics that are saved on a cloud-based dashboard
- Posture support and alignment
- In real-time clinician controls for each leg
- Smart assist software
- Adaptive gait training using built-in sensors
- Pre-ambulatory tools to help with balance, weight shift, squat, and stepping in place
The EksoNR is the first powered exoskeleton cleared by the FDA for ABI. This is in addition to being one of the first wearable robots with FDA approval for spinal cord injury and paralysis due to a stroke. It is capable of supporting people from five feet to six feet and four inches in height, though other limitations may apply.
Mechanically, the EksoNR is a powered hip-knee medical rehabilitation exoskeleton. Taking advantage of being one of the oldest exoskeleton companies in the world, the safety and efficacy of EksoNR are supported by over 100 publications and studies that are listed on the company’s website. As of 2021, there are over 300 EksoNRs distributed in more than 30 countries around the globe.
The EksoNR is a tool to supplement professional physiotherapists, not replace them. It is not available for personal home use. Independent research suggests the use of this exoskeleton allows for a greater number of consistent steps with the appropriate weight shift to be conducted in every physical rehabilitation session.
Exoskeleton Report articles featuring Ekso Bionics (Richmond, CA, USA): Ekso Bionics
USA, website
Exoskeleton Report does not endorse one exoskeleton product over another. The exoskeleton catalog is purely for educational purposes. The catalog is meant to provide an easily accessible birds-eye view of the exoskeleton industry, and a quick method to sort exoskeletons by type and purpose. All prices are approximate and are meant to provide a general sense of the cost of the devices.










Reviews
There are no reviews yet.