Description
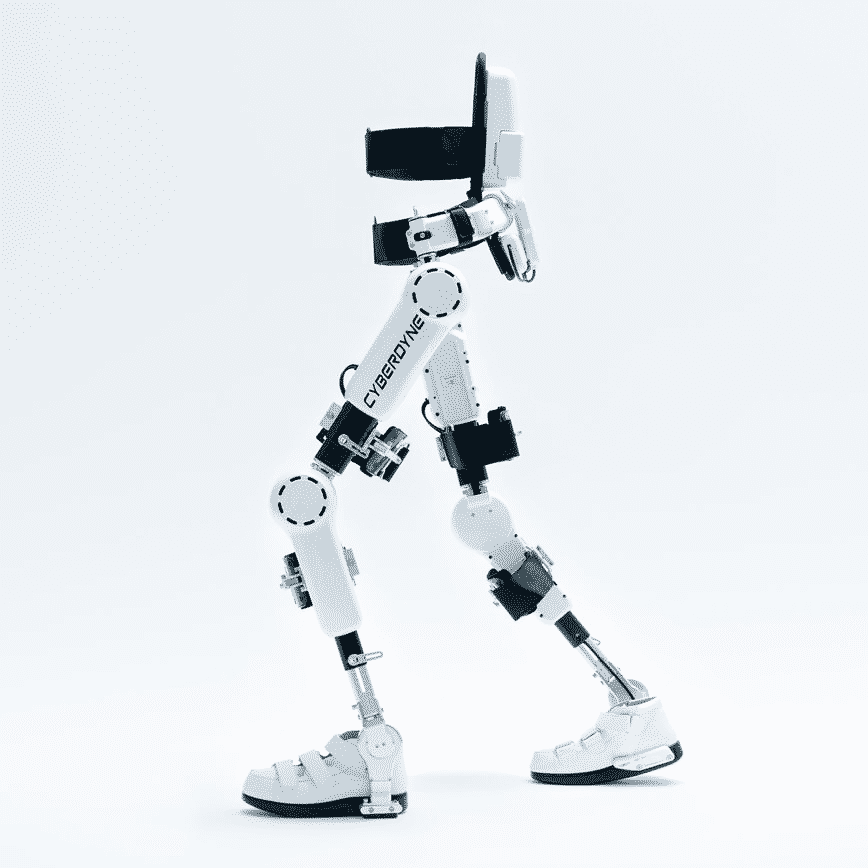
HAL® – Lower Limb Model for Medical Use and Living Support is the flagship product of the Japanese exoskeleton company CYBERDYNE. CYBERDYNE is one of the oldest and the largest dedicated exoskeleton companies. HAL® has been approved for use in the European Union and Japan. HAL®for Medical Use is the first exoskeleton to obtain CE Marking [CE 0197] as a robotic medical device.
Design intent:
- To be used with patients with musculoskeletal ambulation disability: spinal cord injury, traumatic brain injury, brain and neuromuscular system disease, etc…
- Powered hip-knee but extends fully to the ground.
- Bio-electrical signal control.
- Detachable controller for physiotherapists.
The brainchild of professor Yoshiyuki Sankai, the earliest HAL prototype was developed around 1997. This has given the HAL exoskeleton nearly 20 years to mature into a successful medical robot (the current HAL is the 5th iteration of the design).
More information on HAL and the company behind it can be found in our previous CYBERDYNE articles.
CYBERDYNE, 〒305-0818, 2-2-1, Gakuen-Minami, Tsukuba, Ibaraki Prefecture, 305-0818, Japan, website
Exoskeleton Report does not endorse one exoskeleton product over another. The exoskeleton catalog is purely for educational purposes. The catalog is meant to provide an easily accessible birds-eye view of the exoskeleton industry, and a quick method to sort exoskeletons by type and purpose. All prices are approximate and are meant to provide a general sense of the cost of the devices.










Reviews
There are no reviews yet.