Description
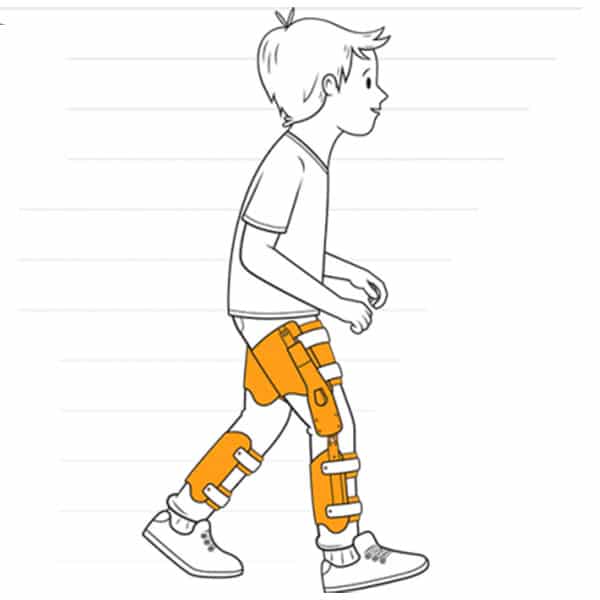
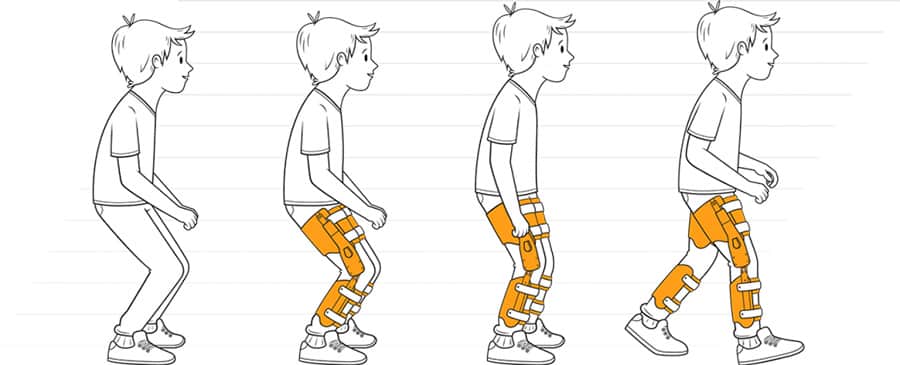
The Agilik (previous version known as Strydr) exoskeleton is a gait rehabilitation solution designed to improve crouch gait, often seen in persons with cerebral palsy, spina bifida, and other knee-extension-deficiency disorders. The intent is to help children retain or increase their mobility, especially for those whose condition will cause further mobility deterioration into adulthood. The goal is to stop or reverse this by reducing crouch gait (or flexed knee), increasing extension, and strengthening the legs.
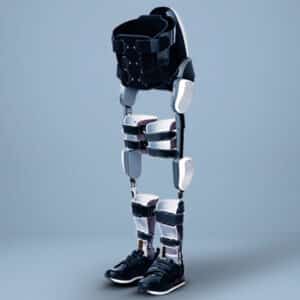
This exoskeleton is lightweight and suitable for children and small adults. Its compact gearbox and motor are designed to apply an assistive torque in the direction of motion to help the muscles or a resistive torque opposing the direction of motion to build strength. The Agilik is used with a custom-molded knee-ankle-foot orthosis (KAFO).
The Agilik has onboard electronics that use a microprocessor and sensors to measure knee angle and speed, as well as initial contact and toe-off. Built-in algorithms analyze the data and construct a gait model, identifying the different regions of gait. Custom torque profiles are created for each specific individual to apply torque across the knee joint, depending on the gait region. The motor applies torque using an integrated motor controller. Gait data is stored in internal memory and can be streamed or uploaded for real-time monitoring and/or review via integrated Bluetooth.
Certifications and third-party testing:
The Agilik is registered as a Class 1 medical device in the IQI category, in both Canada, the US and the EU, where it has the CE mark. Bionic Power is registered with Health Canada and the US FDA as a Class 1 Medical Device Manufacturer.
The Agilik is being used in clinical reviews at the National Institute of Health in Bethesda, Maryland, and The Motion Lab at BC Children’s Hospital in Vancouver, BC.
In a published clinical trial run by NIH researchers, an earlier prototype improved mid-stance crouch gait by 13.3 degrees and initial contact crouch by 6.8 degrees.
Additionally, unpublished testing results using the most recent prototype show significantly improved results.
Geographical Availability:
- North American market
- EU Market
Links to relevant studies or journal papers:
- NIH researchers tested an earlier prototype, and the results are found in this paper: https://www.science.org/doi/
10.1126/scitranslmed.aam9145 - A Pediatric Knee Exoskeleton With Real-Time Adaptive Control for Overground Walking in Ambulatory Individuals With Cerebral Palsy: https://pubmed.ncbi.nlm.nih.
gov/34222356/ - A Robotic Exoskeleton for Treatment of Crouch Gait in Children With Cerebral Palsy: Design and Initial Application: https://pubmed.ncbi.nlm.nih.
gov/27479974/ - Part 2: Adaptation of Gait Kinematics in Unilateral Cerebral Palsy Demonstrates Preserved Independent Neural Control of Each Limb: https://pubmed.ncbi.nlm.nih.
gov/28243195/ - Exergaming with a pediatric exoskeleton: Facilitating rehabilitation and research in children with cerebral palsy: https://pubmed.ncbi.nlm.nih.
gov/28813966/ - The Effects of Exoskeleton Assisted Knee Extension on Lower-Extremity Gait Kinematics, Kinetics, and Muscle Activity in Children with Cerebral Palsy: https://pubmed.ncbi.nlm.nih.
gov/29044202/
Weight of the device:
2.2 kg or slightly less than 5 lb (worn on both legs, and includes all electronics)
Supplied power or torque:
12Nm
Limited to users:
Weight less than 154 lbs (70 kgs).
Age > 5 as the user needs to be able to respond to and act upon requests from a physiotherapist.
For CP patients, GMFCS 1-3
In summary, the Agilik is intended for use to address crouch gait, an inefficient walking gait commonly seen in children with cerebral palsy, spina bifida, and other knee-extension-deficiency disorders. Crouch gait has greater metabolic cost than normal walking gaits and increases pressure on the joints, leading to more joint pain. These factors minimize physical activity, which leads to many of these children losing the ability to walk as they mature and gain weight. The company’s vision is that frequent use of the Agilik will maintain and even improve muscle flexibility, strengthen muscles, and reduce joint pain, in order to maintain the wearer’s independence.
Bionic Power, 2661 Lillooet Street, Vancouver, BC, V5M 4P7 Canada, website
Exoskeleton Report does not endorse one exoskeleton product over another. The exoskeleton catalog is purely for educational purposes. It is meant to provide an easily accessible bird’ s-eye view of the industry and a quick method for sorting exoskeletons by type and purpose. All prices are approximate and are meant to provide a general sense of the devices’ cost.






Reviews
There are no reviews yet.