GOGOA Mobility Robots and CEIT have started the European ALDAK project under COVR. The project is intended to increase safety awareness and ease ATEX...
Category - Standards & Regulations
The ASTM International Exoskeleton Technology Center of Excellence (ET CoE) and Committee F48 on Exoskeletons and Exosuits have created a student paper...
The Japanese exoskeleton developer Innophys announced last quarter that the Muscle Suit Every has become the first exoskeleton assist suit to become ISO 13482...
The ASTM International Exo Technology Center of Excellence (ET CoE) has opened up its doors to research project proposals that can lead to the creation or...
Exoskeletons, exosuits, wearable robots and cobots have lept from research labs and incognito startups to establish a presence on production floors and...
ASTM International is seeking a partnership to launch an exo technology research, development and supporting activities hub. The Center of Excellence (CoE)...
The ReWalk Robotics stroke rehabilitation exosuit, ReStore Exo-Suit, has been cleared by the U.S. Food and Drug Administration (FDA) for sale to rehabilitation...
ReWalk Robotics (Nasdaq: RWLK) best known for developing and offering the ReWalk walking assist exoskeleton is on track to become even more famous with a new...
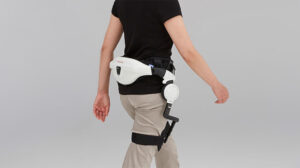
The Spanish exoskeleton manufacturer GOGOA has made history by becoming the first European company to receive CE clearance for its powered lower body HANK...
The Honda Walking Assist Device has earned the European Union (EU) CE certification mark for medical devices. This allows the Honda Motor Company to market...