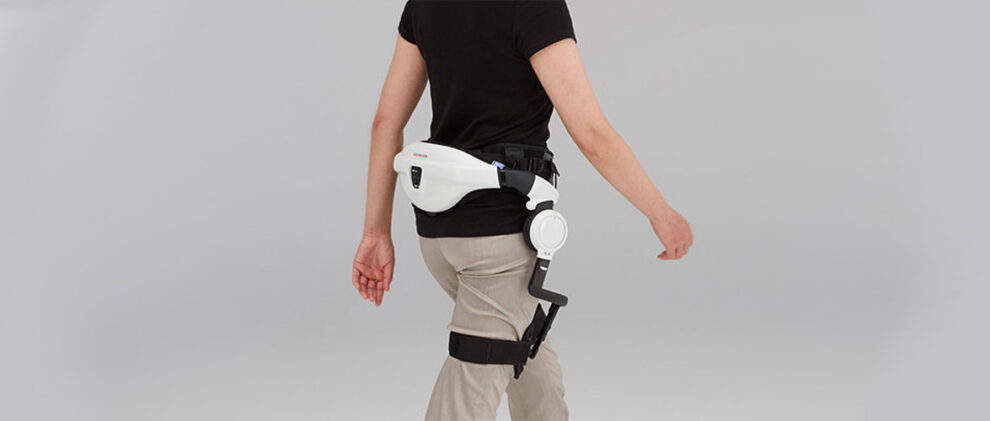
The Honda Walking Assist Device has earned the European Union (EU) CE certification mark for medical devices. This allows the Honda Motor Company to market and commercialize its wearable robot as a medical device in EU countries and signifies conformity with EU standards. The Honda Walking Assist Device is an assistive powered hip exoskeleton for training and rehabilitation of walking.
The Honda Walking Assist Device falls under the Medical Devices Directive (MMD) certification. The MMD approval was accomplished using a cooperative approach between the Japan Quality Assurance Organization and TÜV Nord Cert GmbH, respectively a Japanese and a German third-party ISO certifiers. Both organizations leveraged the content of ISO 13482.
A Big Win for Standards!

This is a perfect example of how the international standardization work for exoskeletons can lead to certification in large markets such as the EU. One major advantage that the Honda Walking Assist Device had going into its CE mark certification was that it was already certified in October 2015 under ISO 13482. ISO 13482 itself was issued by the International Organization for Standardization (ISO) in 2014 and is the only international safety standard for personal care robots, which was published by what is now a familiar group within the exoskeleton industry: ISO/TC 299 Robotics.
The above is a clear example of how the standardization efforts of JIS, ASTM, ISO, and WearRA can lead to an accelerated path to larger markets. Click here for coverage of recent efforts in the field of exoskeleton standards. In 2018, the work is continuing at full speed:
- ISO/TCC 299 Work Group 2 and 4, which have the largest wearable robotics presence is meeting Jan 29th – Feb 1st
- ASTM F48 Exoskeletons and Exosuits will have its first face-to-face meeting Feb 13th-14th
- WearRAcon18 will take place March 21st-23rd
Honda’s Rich History in Exoskeleton Development
Between 1999 and 2008, the Honda Fundamental Research Institute created at least 30 wearable robot prototypes. This is the same group that created the ASIMO humanoid robot. Towards the end of 2008, there were two final models left. Grossly simplified, the Honda researchers split walking into two problems: fighting against gravity to maintain standing and generating a force to move forward. Thus the Honda Body Weight Support Assist and the Honda Walking Assist devices were born.
Unfortunately, the Honda Body Weight Support Assist has not been seen in the news for years. It can provide the equivalent of 6.5 to 18 pounds pushing up on a seat, depending on the bend of the knees and the force detected by the sensors in the shoe soles. Perhaps it was too difficult for people to wear comfortably without training. Ten years later, we now know that many of the gait assist devices require multiple sessions for the user to feel in control.
After 19 years of work, the CE marking of approval for the Honda Walking Assist Device is a well-deserved achievement for the company. The collective efforts in exoskeleton standards development also played a critical role in reaching this milestone.
Sources:
Honda Walking Assist Obtains Medical Device Approval in the EU, Honda News Release – Power Products, January 18, 2018, http://world.honda.com/news/2018/p180118eng.html
Walking Assist – Introduction, How it Works, Structure, Features, Honda, Accessed January 2018, http://world.honda.com/Walking-Assist/introduction/index.html
ISO 13482:2014 Robots and robotic devices — Safety requirements for personal care robots, International Organization for Standardization, Accessed January 2018, https://www.iso.org/standard/53820.html
Test Drive: Honda Stride Management Assist, Bodyweight Support Assist [Update]. TechCrunch, Peter Ha, April 14th, 2009, https://techcrunch.com/2009/04/14/test-drive-honda-stride-management-assist-bodyweight-support-assist/
Honda Walking Assist Presentation, Crunch Gear, YouTube, April 14th, 2009, https://www.youtube.com/watch?v=7YP12–RBfY
Why Design Now?: Bodyweight Support Assist, Cooper Hewitt, YouTube, April 23rd, 2010, https://www.youtube.com/watch?v=SOP4cc6c78M







I would like to know more about the cost and if they are available in the United States.
Hi Joyce, the answer to both questions is difficult to tell at this point. If there are some units in the U.S. they will most likely be purely for testing. I don’t have any new information on the price either, sorry.
Extremely interested in getting more information on
The availability in Canada. I have sIBM Disease and
would like to see if it would be suitable for me to use.
I would volunteer to test this product in Canada.
Unfortunately, we don’t have that information.
I’d like to know about this device for reasons could really use this after having a stroke it left me not able to 2alk well but when I saw this on the web it was awesome and it would be great for me my name is Audrey I’d like some info please
Dear Audrey, unfortunately, Honda can be very secretive about its projects. As of this writing this comment, the Exoskeleton Report team knows about as much as you do. Stay tuned though and we will post more information as it becomes available.
Had a mild celbrel stroke learning to walk again,price can rent? Will it help?going on 9 months.please send info.( Ischemic stroke)
Dear Frank, there has been quite a bit of interest regarding the Honda Walking Assist but at this time the ExR team doesn’t know if it can be rented and if so where. Stay tuned as we will post any new developments.
Would like to know when it will be available for use by persons with disability in Malta europe. I have weakness in my legs due to mascular dystophy.
Dear Joe,
Unfortunately, our team doesn’t have the answer to this quite yet. This Honda device has sparked significant interest.