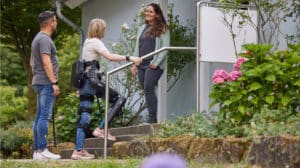As of March 2023, 11 medical exoskeletons have been approved for walking, gait assistance, and physical rehabilitation by the US FDA. The FDA classifies...
Tag - FDA
ReWalk Robotics has overcome a major regulatory hurdle by having the first medical exoskeleton to have FDA approval for assisting with walking up stairs and...
The ReWalk Robotics stroke rehabilitation exosuit, ReStore Exo-Suit, has been cleared by the U.S. Food and Drug Administration (FDA) for sale to rehabilitation...
The Japanese exoskeleton manufacturer and one of the largest exoskeleton companies in the world, CYBERDYNE Inc., has obtained marketing clearance from the U.S...
After many years, the HAL – Lower Limb Exoskeleton for Medical Use by CYBERDYNE Inc. has finally arrived in the U.S. HAL stands for Hybrid Assistive...
“Basically, I’m here to announce we’re building Iron Man,” President Obama told the press in 2014. Yet while bold claims are being made...
The Department of Health and Human Services, Food and Drug Administration (FDA) announced on February 24th, 2015 that all powered exoskeletons will be...