As of March 2023, 11 medical exoskeletons have been approved for walking, gait assistance, and physical rehabilitation by the US FDA. The FDA classifies medical-powered exoskeletons as Class II devices, code PHL, with the exact definition as: “A powered exoskeleton is a prescription device that is composed of an external, powered, motorized orthosis that is placed over a person’s paralyzed or weakened lower extremity limb(s) for medical purposes.”
The 11 medical exoskeletons are in no particular order:
Keeogo Dermoskeleton System by B-Temia, powered knee exoskeleton
- HAL for Medical Use (lower limb type) by CYBERDYNE, powered hip-knee exoskeleton
- EksoNR by Ekso Bionics powered hip-knee exoskeleton
- ExoAtlet-II by ExoAtlet Asia, also a powered hip-knee exoskeleton
- Honda Walking Assist Device, Honda Motor Company, powered hip exoskeleton
- Indego, formally by Parker-Hannifin Corporation, recently acquired by EksoBionics, powered hip-knee exoskeleton
- ReWalk by ReWalk Robotics, powered hip-knee exoskeleton
- ReWalk ReStore by ReWalk Robotics, powered ankle exosuit
- GEMS-H by Samsung Electronics, powered hip exoskeleton
- Phoenix by Ottobock, formally suitX (which itself was formally US Bionics), is a powered hip exoskeleton with passive knee support
- Atalante by Wandercraft SAS powered hip-knee-ankle exoskeleton
As of March 2023, only the ReWalk exoskeleton is approved for use with stairs and curbs by the FDA (see: link). Only the ReWalk Personal and Indego are approved for a home or personal use. All devices can be used in a rehabilitation center environment. The Atalante is the only self-balancing exoskeleton approved by the FDA (though the REX may join the list soon). The remaining devices either have optional or required additional mobility aids, such as crutches or a walker. There are several devices, like the Atalante, that can potentially be used by adolescents (but there are no pediatric exoskeletons on this list). All operators must complete a training program prior to utilizing the exoskeletons.
Some of the FDA-approved powered exoskeletons have specific fall protection and mitigation considerations, specifically:
- Atalante: must be used in combination with a safety rail.
- ReWalk must be used with crutches. The Indego, the ExoAtlet-II (and presumably the Phoenix), should be used with crutches or a walker, while the EksoNR can be used with crutches, a walker, or a cane.
- The HAL for Medical Use has to be tethered to a body weight support system (usually overhead).
- All but the home-use versions of the ReWalk and the Indego have been cleared for use only under the supervision of a trained healthcare professional.
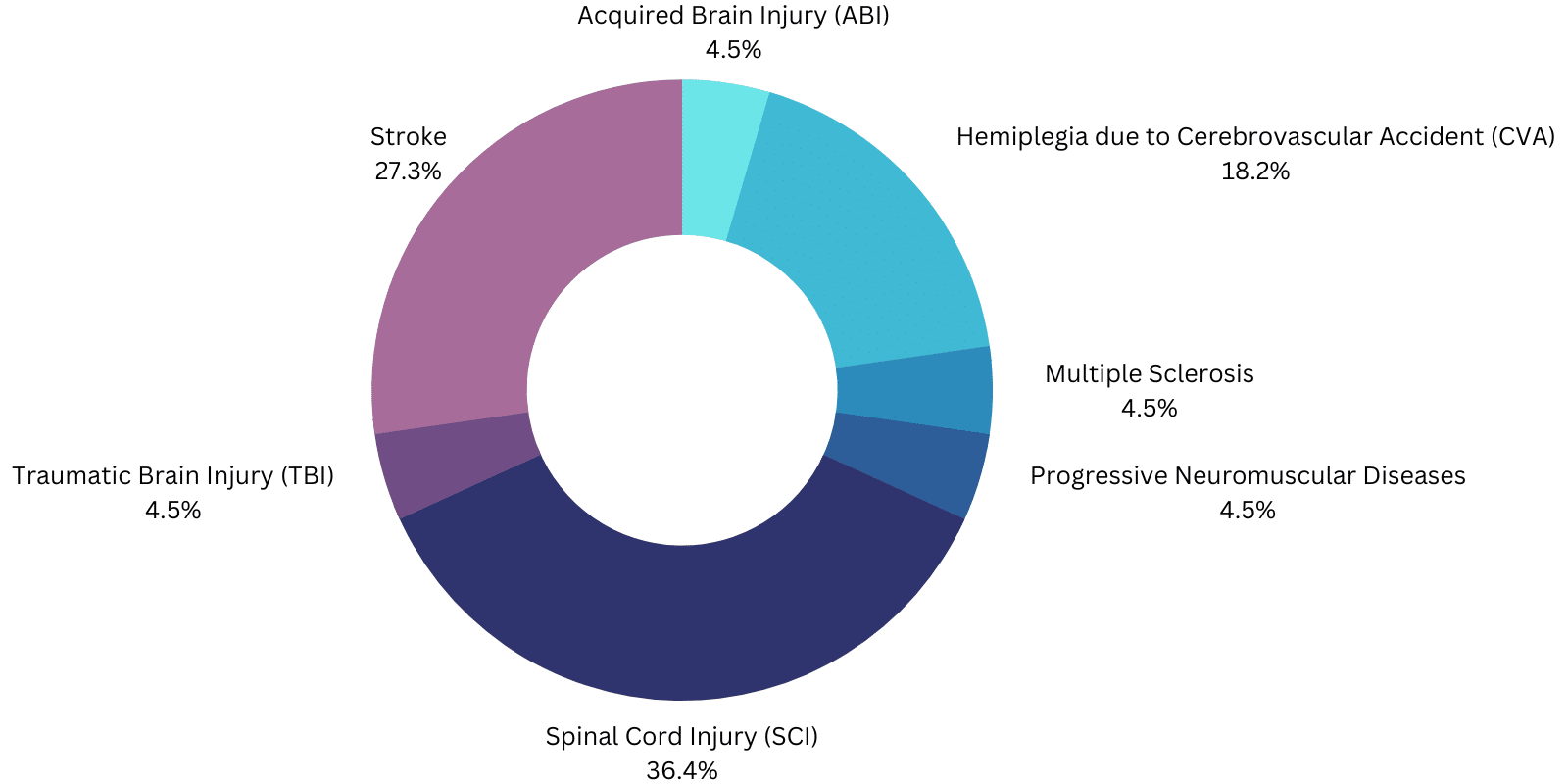
The Intended Ese Case for the FDA-Approved Powered Exoskeletons is to Assist With Ambulation for Patients/Users With:
- ExoAtlet-II is for the functional rehabilitation of individuals under the supervision of a trained physical therapist with upper extremity motor function of at least 4/5 in both arms who have spinal cord injuries (SCI) T4 to L5 or C7 to T3 (ASIA D).
- Phoenix: T4-L5 level spinal cord injury (SCI).
- Atalante: hemiplegia due to cerebrovascular accident (CVA).
- GEMS-H: Individuals with stroke who have gait deficits and exhibit gait speeds of at least 0.4 m/s and are able to walk at least 10 meters with assistance from a maximum of one person.
- ReWalk: SCI T7 to L5 personal use with constant supervision or SCI T4 to T6 for physical rehabilitation at a dedicated institution.
- ReWalk Restore: should be “used to assist ambulatory functions in rehabilitation institutions under the supervision of a trained therapist for people with hemiplegia/ hemiparesis due to stroke who can ambulate at least 1.5m (5ft) with no more than minimal to moderate levels of assistance.”
- Indego: ambulatory assistance for SCI levels T3 to L5 for personal use with supervision and SCI at levels C7 to L5 in rehabilitation institutions or hemiplegia due to CVA with motor function 4/5 in at least one upper extremity.
- Honda Walking Assist: “Individuals with stroke who have gait deficits and exhibit gait speeds of at least 0.4m/s and are able to walk at least 10 meters with assistance from a maximum of one person” in rehabilitation institutes.
- EksoNR: “The EksoNRm is intended to perform ambulatory functions in rehabilitation institutions under the supervision of a trained physical therapist for the following populations:
-
- Individuals with multiple sclerosis (upper extremity motor function of at least 4/5 in at least one arm).
- Individuals with acquired brain injury, including traumatic brain injury and stroke (upper extremity motor function of at least 4/5 in at least one arm).
- Individuals with spinal cord injuries at levels T4 to L5 (upper extremity motor function of at least 4/5 in both arms).
- Individuals with spinal cord injuries at levels of C7 to T3 (ASIA D with upper extremity motor function of at least 4/5 in both arms).
- HAL for Medical Use: is for those who exhibit sufficient residual motor and movement-related functions of the hip and knee to trigger and control HAL and
- with spinal cord injury at levels C4 to L5 (ASIA C, ASIA D) and T11 to L5 (ASIA A with Zones of Partial Preservation, ASIA B);
- post-stroke paresis
- paraplegia due to progressive neuromuscular diseases (spinal muscular atrophy, spinal and bulbar muscular atrophy, amyotrophic lateral sclerosis, Charcot-Marie-Tooth disease, distal muscular dystrophy, inclusion body myositis, congenital myopathy, muscular dystrophy)
- Keeogo Dermoskeleton System is for individuals “with stroke who have gait deficits and sufficient hip
(MMT Hip ≥ 3) and knee strength (MMT Knee ≥ 2) and who are capable of standing and initiating gait movement without assistance.”
Reported User Height and Weight limitations:
| Device: |
Height Range of User
|
Weight Range of User |
| GEMS-H | 1.55 m to 1.91 m | 45 tp 100 kg |
| Atalante | 1.60 to 1.90 cm | up to 90 kg |
| Phoenix | 1.60 m to 1.87 m | up to 91 kg |
| ReWalk | 1.60 m to 1.90 m | up to 100 kg |
| ReWalk Restore | 1.42 m to 1.92 m | up to 120 kg |
| Indego | 1.50 m to 1.90 m | up to 113 kg |
| Honda Walking Assist | 1.4 m to 2.0 m | up to 100 kg |
| ExoAtlet-II | 1.60 m to 1.90 m | up to 100 kg |
| EksoNR | 1.58 m to 1.88 m | up to 100 kg |
| HAL for Medical Use | 1.50 m to 1.90 m | 40 to 100 kg |
| Keeogo Dermoskeleton System | 1.52 m to 1.88 m | up to 130 kg |
If you see any errors or would like an article on a specific topic, don’t hesitate to reach out using the ExR Contact Form. Featured image (above): Demonstration of the Ekso GT by Shane Mosko and Physical Therapist Jenn Macievich at Ekso Bionics HQ, Richmond, 2016 (background removed).
Source:
- Product Classification, FDA, Accessed on March 2023, link
- Palermo AE, Maher JL, Baunsgaard CB, Nash MS. Clinician-Focused Overview of Bionic Exoskeleton Use After Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2017 Summer;23(3):234-244. doi: 10.1310/sci2303-234. PMID: 29339899; PMCID: PMC5562031.
-
Evaluation of Safety and Performance of the Atalante System With Patients With Lower Limb Paralysis, NIH, 2018, link









Add Comment