Exoskeleton Report strives to stay ahead of new trends, bringing you the latest in wearable robotics and exoskeleton technology. Today, we’re excited to...
Tag - Classification
Last week the 11th ASTM International Committee F48 on Exoskeletons and Exosuits meeting was held in conjunction with ASTM committee week this April in Denver...
What is an exoskeleton? The field of exoskeleton systems is continuously evolving and re-inventing itself, so it is still difficult to create a singular...
NIST and the U.S. Army NSRDEC are holding a two-day meeting with experts from the industrial, military, and medical communities to discuss exoskeleton...
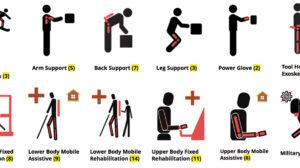
Catalog of commercial wearable exoskeletons: Target Audience Criteria Help us make this complete Other catalogs Prior work Categories, keywords, and companies...
The Ekso GT by Ekso Bionics has finally been cleared by the FDA for clinical use. The company received clearance to market its exoskeleton for use in...
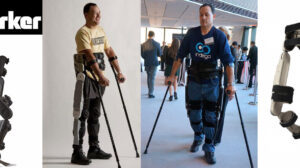
There is a great disturbance in the exoskeleton industry. We have a new exoskeleton approved by the FDA for personal use: The Indego by Parker Hannifin. The...
The field of exoskeleton devices stands to greatly gain from 3rd party testing. Manufacturers and sellers of exoskeletons can build a stronger case for the...
The first Wearable Robotics Association Convention, WearRAcon16 is in full swing in its second and busiest day. Exoskeleton experts from around the world...
The HAL lower limb rehabilitation exoskeleton for medical use (HAL® for Medical Use) has been approved to be manufactured and sold as a Medical Device by the...