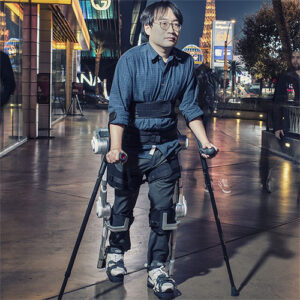
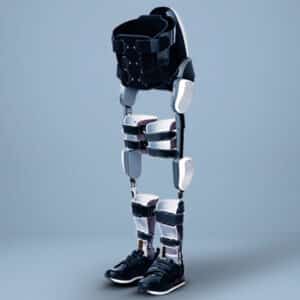
Description
ExoBand is engineered to facilitate efficient walking with minimized energy expenditure. This innovative technology operates passively, leveraging advanced elastic technology to efficiently conserve and optimize energy usage. Its design integrates functional and comfortable fabric, ensuring seamless incorporation into daily routines. With a lightweight construction free from complex gears and batteries, ExoBand enhances mobility without compromising comfort or practicality. Furthermore, its compatibility with existing walking aids enhances versatility and usability. Representing a significant stride in assistive technology, ExoBand combines functionality and comfort to empower individuals seeking greater mobility independence.
Inspired by biomechanical principles, the design endeavors to amplify walking capabilities, enabling prolonged and improved ambulation. The approach has been refined through meticulous observation of individuals afflicted with diverse pathologies aiming to enhance deamblulation and stability. ExoBand embodies deliberate simplicity, underpinned by three years of exhaustive research and development, alongside the creation of numerous prototypes. Immediate benefits of ExoBand utilization include trunk stabilization, posture enhancement, and reduced fatigue. Additionally, post-rehabilitation program implementation yields significant improvements, such as a 20% average increase in walking distance.
Pediatric version: the ExoBand comes with a pediatric version called ExoBaby. It is functionally identical to the adult version. More information can be found on the Moveo website.
Weight of the device:
1 lb (0.5 kg)
Certifications and/or 3rd party testing results:
- ExoBand is a class 1 medical device CE marked, compliant with the essential requirements and safety standards outlined by the European Union (EU).
- In compliance with FDA regulations outlined in FDA 21 CFR 820, ExoBand is compliant with the quality system requirements established by the U.S. Government.
- ExoBand received the Prix Galien 2021 award for the best medical device.
- ExoBand is a patented device.
Regions and or countries where the exoskeleton can be purchased?
ExoBand can be purchased in EU, UK, USA.
Links to relevant studies or journal papers that may feature this exoskeleton?
You can find indexed journal publications as well contributions to conferences on the “Scientific Research and Clinical Suties” section of the Moveo website: link
Links to user testimonials:
More testimonials on the “experiences” page from Moveo’s website: https://www.moveowalks.com/en/experiences/
Limited to users who are (age, weight, height, etc…)
ExoBand is suitable for individuals who are capable of walking, particularly those with a minimum range of hip extension.
Moveo s.r.l. – Via Monsignor Fortin, 38, Padova, Veneto 35128, IT – website
We partner with companies we believe in. Some links on the Exoskeleton Report are affiliate links, which means we may earn a commission if you purchase through them. This helps support our work and keeps the site running.
Exoskeleton Report does not endorse one exoskeleton product over another. The exoskeleton catalog is purely for educational purposes. The catalog is meant to provide an easily accessible birds-eye view of the exoskeleton industry, and a quick method to sort exoskeletons by type and purpose. All prices are approximate and are meant to provide a general sense of the cost of the devices.








Reviews
There are no reviews yet.