Description
Emovo Assist is a hand exoskeleton designed to support users with motor disabilities at home and during activities of daily living.
It is built by Emovo Care, a startup based on technologies initially developed at the École Polytechnique Fédérale de Lausanne (EPFL) in Switzerland.
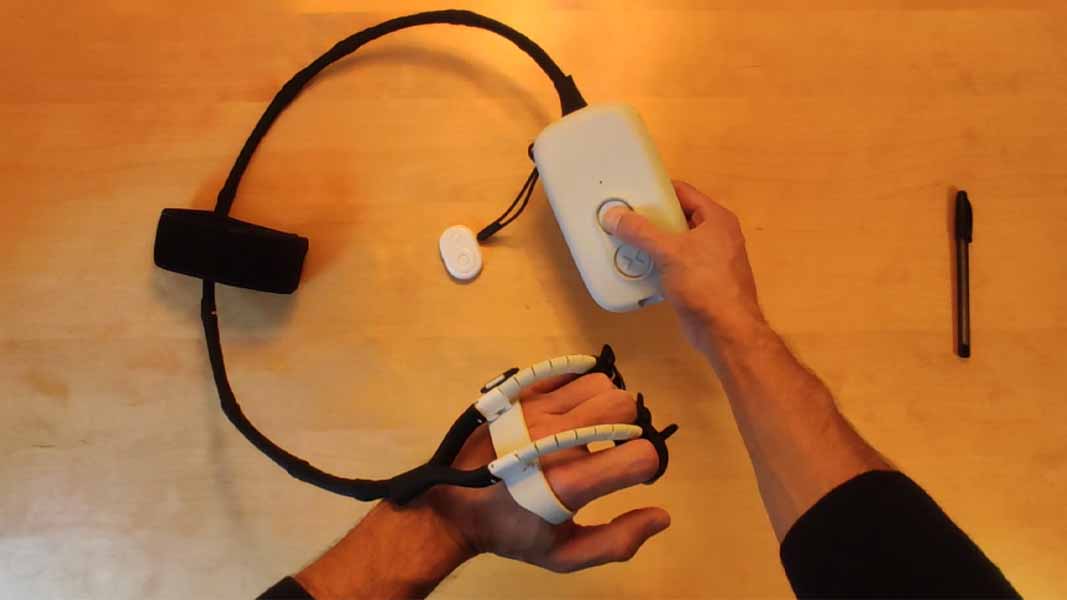
The exoskeleton consists of three parts:
– the actuation module: It includes the battery pack, motors, and input buttons. It can be handheld or worn at the waist.
– the tendons: 2 to control flexion and extension of 4 fingers (or 3 for the version with also thumb actuation).
– the hand interface: A partial glove that connects the tendons to the fingers through various anchoring points.
Portability, wearability, and ease of use are key aspects in the design of the Emovo Assist, which comes in a compact package (490 g) and can be worn and used autonomously by patients. The exoskeleton lays on the dorsal side, allowing also clenched hand patients to use the exoskeleton.
At the moment, two patents protect the technology and one clinical study shows the capabilities of the initial prototype.
Randazzo, Luca, et al. “mano: A wearable hand exoskeleton for activities of daily living and neurorehabilitation.” IEEE Robotics and Automation Letters 3.1 (2017): 500-507.
The current version of the exoskeleton can be operated using two buttons on the actuation module, which allow to open and close the hand. The system has been tested with automated open-close cycles and various input controls, as EMG and EEG.
The device can actively support hand opening and closing in a fully portable system, which is autonomously wearable and usable by patients themselves.
By providing active assistance in daily scenarios, Emovo Assist aims at accelerating motor recovery through intensive and meaningful use.
Links to testimonials, independently produced videos by users or integrators, etc…:
Certifications and/or 3rd party testing results:
EN ISO 13485:2016, Certificate#: MD 736347
Weight of the device:
490 g
Supplied power or torque:
36 W
Limited to users who are (age, weight, height, etc…)
The first iteration of Emovo Assist will specifically target hemiparetic stroke survivors with typical anthropometric features (45~60 years old, 50~90 kg, 1.60~1.90 m tall)
Emovo Care SA, EPFL Innovation Park, Bâtiment C, 1015 Lausanne, Switzerland, website
Exoskeleton Report does not endorse one exoskeleton product over another. The exoskeleton catalog is purely for educational purposes. The catalog is meant to provide an easily accessible birds-eye view of the exoskeleton industry, and a quick method to sort exoskeletons by type and purpose. All prices are approximate and are meant to provide a general sense of the cost of the devices.
This exoskeleton catalog entry has been made with the assistance of Stefano Carisi, MS in BioRobotics and Biomechanical Design from the Delft University of Technology an experienced product manager in human-machine collaboration.







Reviews
There are no reviews yet.