This article was originally posted on Forbes.com (link) on August 16, 2022. A passive exoskeleton model designed and evaluated with the help of over 100...
Category - Business
Exoskeleton business news and articles.
Many North American exoskeleton developers owe their beginnings to U.S. Department of Defense (DoD) investments. Yet, no military exoskeletons or wearable...
The Japanese pioneer of wearable robotics and exoskeleton technology, ATOUN Inc, is closing its doors. The company will officially dissolve at the end of April...
Exoskeleton giant Sarcos acquires fellow robotics company RE2, but also delays the Guardian XO Beta.
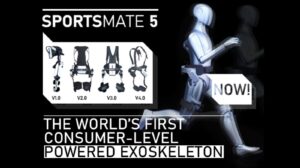
The successful Kickstarter of the SPORTSMATE 5 demonstrates that there is a strong interest in powered commercial exoskeletons that can be purchased by anyone...
The global powerhouse in prosthetics manufacturing, Ottobock, is now set to become a world leader in exoskeleton technology after it acquired a 100% stake in...
Sarcos Robotics’ business combination with Rotor Acquisition Corp has been approved by Rotor Shareholders. Rotor will change its name and the combined...
Polish Bionics, founded by Jędrzej Stranz, is the latest company to bring exoskeleton technology to the popular crowdfunding website Kickstarter. The founder...
Myomo, INC (NYSE: MYO) released its financial results for the three and six months ending June 30, 2021. In Q2 2021, the company realized a 262% increase in...
ReWalk Robotics, one of the leaders in medical powered exoskeletons and exosuits, has released its three-month and six-month updates for 2021. The company...