The ASTM Committee F48 on Exoskeletons and Exosuits will hold its first in-person meeting Tuesday and Wednesday, February 13-14th, 2018. The meeting will be...
Category - Standards & Regulations
Attention, there is a new testing and standards group in town, the ASTM Committee F48 on Exoskeletons and Exosuits! Curious what ASTM aims to accomplish...
There are major developments in defining how to communicate and examine wearable robotics and exoskeleton technology. This also presents an opportunity for...
How are exoskeletons tested and evaluated numerically? The principle engineers from Ekso Bionics, one of the oldest companies in the exoskeleton industry, set...
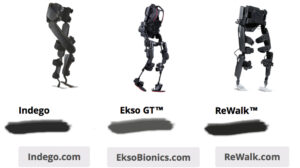
The Department of Veterans Affairs Palo Alto Health Care Systems (VAPAHCS) has submitted a request for an unidentified number of Ekso GT exoskeletons and a...
What is the shape of the ever growing exoskeleton industry in 2016 and what does the term mean in the first place? In 2016, exoskeleton industry is growing at...
The field of exoskeleton devices stands to greatly gain from 3rd party testing. Manufacturers and sellers of exoskeletons can build a stronger case for the...
The Department of Health and Human Services, Food and Drug Administration (FDA) announced on February 24th, 2015 that all powered exoskeletons will be...