The ASTM Committee F48 on Exoskeletons and Exosuits will hold its first in-person meeting Tuesday and Wednesday, February 13-14th, 2018. The meeting will be held in proximity to the ASTM Headquarters at the Philadelphia Marriott West; West Conshohocken, PA US.
F48 on Exoskeletons and Exosuits
The drive behind F48 is to create terminology, testing and safety standards for exoskeletons and exosuits. This process can be allowed to happen naturally, but the main risk is that medical, industrial, military, and commercial/recreational exoskeleton developers will create various and possibly contradictory sets of terms and requirements which would be difficult to consolidate down the road.
The Committee Structure currently consists of:
F48.01 Design and Manufacturing
F48.02 Human Factors and Ergonomics
F48.03 Task Performance and Environmental Considerations
F48.04 Maintenance and Disposal
F48.05 Security and Information Technology
F48.91 Terminology
ASTM Balancing
ASTM structures and binds the main committee and all of the subcommittees by internally voted on bylaws. Voting and voting objections are recorded and handled using an online system. Any objections or negative votes are permanently stored as is their resolution.
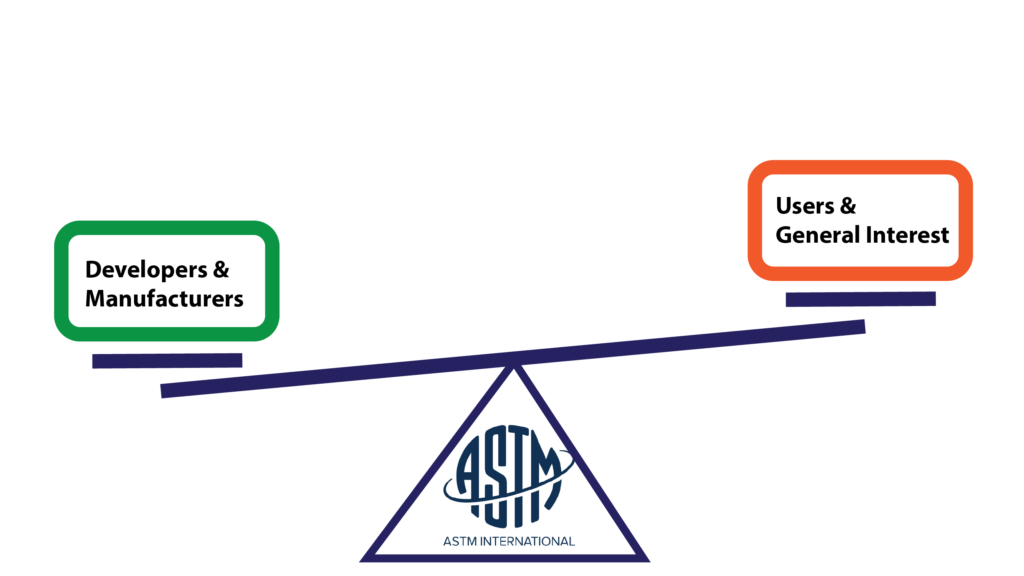
ASTM recognizes the danger of having an unbalanced group of contributors. While everyone is allowed to participate and voice objections, the voting block has to be balanced between producers and users. Standards made only by developers risk becoming inaccessible to users, while standards made entirely by users could become more of a wishlist than a useful tool.
Device Similarity
The F48 meeting in February is an opportunity to discuss how to distinguish exoskeletons that look almost identical to each other. Until recently, most exoskeletons had some features that could easily distinguish them from one another. Take for example the analysis by the Department of Veterans Affairs Palo Alto Health Care Systems in which they compare the Indego, EksoGT, and ReWalk (Sep 2016). Each of the powered exoskeletons had many unique, distinguishing features. The Indego incorporates FES (Functional Electrical Stimulation), the EksoGT utilizes SmartAssist control software, and the ReWalk has to the most extensive home use history and has renown device ruggedness.
With the proliferation of wearable technology, interested parties and potential buyers now face much harder choices in selecting wearable solutions. One example of device convergence can be seen in the three most popular shoulder support exoskeletons that attach between the shoulder and elbow: the Levitate AIRFRAME™, the Ekso Bionics EksoVest, and the suitX shoulderX (see arm support industrial exoskeletons). When a consumer or an investor looks at one of the three devices should they be forced to learn the terminology of each company and perform their own evaluation of each device?
The Face to Face Meeting for the ASTM Committee F48 on Exoskeletons and Exosuits in February 2018 will take exoskeletons one step closer to being as clearly defined as buying a car. A car crash safety rating means the same thing for Honda, Ford, BMW and everything between.
Meeting Details:
Pre-registration for the first Face to Face meeting of the F48 Committee since its organization last year is now open. Dates: Feb/13-14, 2018 @ ASTM HQ – Please, visit the ASTM page (link) for registration, hotel information, committee fees, additional information, schedule, and contact information for questions:
This meeting is open to all interested parties, however, registration is required.
More on Standards:
In 2018 the exoskeleton industry will likely be dominated by standards, testing, and terminology development. Looking back:
- 2015: Scores of companies went public with their projects. The main sentiment was “I had no idea there were others working on this.” See: 2015 Year in Review: Exoskeletons and Wearable Robotics
- 2016: Most exoskeleton developers became much more comfortable talking publicly about their projects. A clear separation between real wearable devices and those seen is science fiction was made. For more read: 2016 Year in Review: Exoskeletons and Wearable Robotics
- 2017: Last year, the focus shifted to justifying exoskeletons, exosuits and wearable robotics. Questions like what is the return on investment (ROI), how can the device be tested, is it safe, etc… for the first time began dominating discussions. This was in sharp contrast to previous years were the main focus of conversations was discovering the technical limits of the technology.
In 2018, the exoskeleton industry has evolved to the point where most companies have presented their products and have made specific claims regarding their capabilities. However, each company describes their products differently, and there are no agreed upon quantifiable tests that similar devices can be subjected to for comparison. The F48 meeting presents an opportunity to tackle these issues.
Update: That’s not all! ISO meeting in January:
In addition to the ASTM F48 meeting in February and WearRAcon18 in March, NIST will also host a gathering of ISO/TCC 299 Robotics. The first ISO workgroup with relevance to wearable robotics is WG 4 – Service Robotics which will meet on Monday and Tuesday, Jan 29-30, 2018. Another one is WG 2 – Personal Care Robot Safety which will meet Jan 30 – Feb 1, 2018. The meeting will be held at the NIST headquarters in Maryland, US. For additional information and registration visit Nist.gov








Add Comment